Abstract
Background: Acute myeloid leukemia (AML) is the most common leukemia in adults with more than 20,000 new cases in the U.S. each year. While about two-thirds of patients who receive standard induction chemotherapy reach complete remission, about half of those will relapse within two years. There are several known risk factors for relapse including age, cytogenetics, and a few specific mutations; however, there is still a need for better prognostic information to guide therapy and improve patient outcome.
Measurement of residual disease has proven helpful in predicting patient outcome. Current approaches for measuring minimal residual disease (MRD) include multiparameter flow cytometry, which can detect abnormal myeloid blast populations based on the expression of cell-surface markers, and targeted molecular assays measuring variant frequency of common mutations. Molecular approaches have higher sensitivity than flow cytometry; however, many targeted assays follow only a single mutation, which does not capture the within patient genomic heterogeneity of AML.
In this study, we applied whole genome sequencing (WGS) to study MRD at the time of complete remission in AML, revealing the full spectrum of genomic response to treatment and its correlation with patient outcome.
Methods: Bone marrow biopsy or peripheral blood specimens were collected at the time of presentation and remission from 18 patients with AML. All patients were treated with standard-of-care induction chemotherapy. Among the 18 patients, 8 relapsed and 10 did not relapse. Corresponding clinical and outcome data (median follow-up of 26 months) were collected as well as matched flow cytometry data at remission on 11 of 18 cases.
Deep whole genome sequencing (104x median depth) was performed on DNA from paired presentation and remission specimens. Single nucleotide variants were called from both samples, with a mutation caller capable of identifying low frequency variants. Quality filters and population frequency filters were applied to the variant set. Remaining variants were then classified into five classes based on their variant frequency pattern.
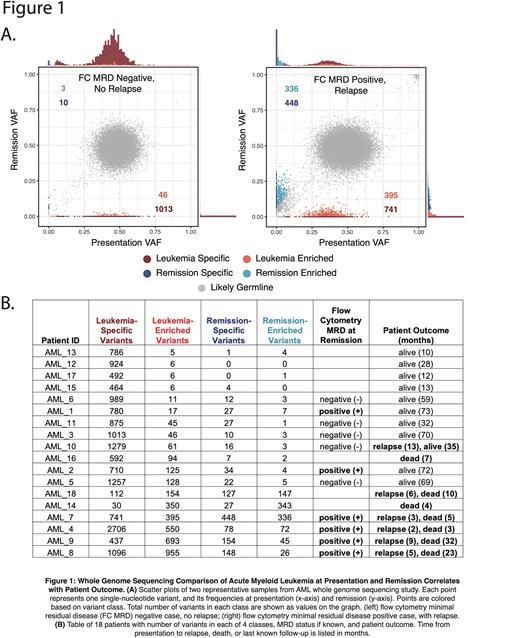
Results: Figure 1A shows variant frequencies for two representative patients. Variants can be broadly classified into five classes: (A) likely germline variants, clustered at the expected variant frequency of 0.5 (grey); (B) leukemia-specific variants which are dramatically reduced in frequency upon treatment (dark red); (C) leukemia-enriched variants which are incompletely reduced at remission (bright red); (D) remission-specific variants that were not detectable at presentation but are prevalent at remission (dark blue); and (E) remission-enriched variants which have increased in frequency at remission (light blue).
The proportion of variants belonging to classes B, C, D, and E correlates with both MRD status and patient outcome, as shown in Figure 1B. Patients with a higher ratio of leukemia-specific to leukemia-enriched variants are more likely to be alive at one year. Patients who relapsed tend to have a higher number of leukemia-enriched variants, which may represent variants in pre-leukemic cells or leukemic stem cell populations. Patients with a poor outcome also tend to have a higher number of new and increasing remission-specific and remission-enriched variants. This suggests that their remission state has not returned to normal, but is re-populated with clonal populations of cells containing a significant number of somatic variants.
The few discrepancies between WGS and MRD flow cytometry suggest that the WGS data could be more predictive of patient outcome. For example, there are two MRD positive cases in which the WGS profiles suggest a good outcome, consistent with the actual patient outcome. In two other cases, neither flow cytometry nor WGS profiles predicted the patients’ relapses.
We find that DNMT3A, TET2, ASXL1 mutations have high frequencies at both time points and cluster outside of the labeled classes of variants, whereas other driver gene mutations such as NPM1, NRAS, and IDH1 cluster with the leukemia-specific variants.
Conclusion: Our study shows promising evidence for the utility of WGS to predict patient outcome in AML in the setting of intensive chemotherapy. Combining WGS with targeted deep sequencing approaches may further increase the predictive power of genomic approaches. Collection and analysis of a larger case series is ongoing.
Disclosures
Allen:BMS: Current equity holder in publicly-traded company; C4 Therapeutics: Current equity holder in private company; Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Research Funding. Levine:Imago, Mission Bio, Bakx, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Ajax, Abbvie, Constellation, Zenalis, Celgene, Roche, and Prelude: Other: research support; Syndax, Incyte, Janssen, Astellas, Morphosys and Novartis: Consultancy; Gilead and Novartis: Other: Grant reviews; Astra Zeneca and Kura: Other: honoraria for invited lectures ; Qiagen: Other: supervisory board member.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal